
If you’ve ever measured the pH of your swimming pool, you understand that a proper pH range is important for anyone going into water. You wouldn’t want to swim in your own pool if the pH was not in a safe range for you. It is no different for the plants and animals that make their homes in the oceans.
Let’s break down pH and its relation to the ocean for a minute. pH is a measurement of the acidity of a solution. Acidity refers to the amount of hydrogen (H+) ions in the solution—the more H+ ions there are in a solution, the higher the acidity. Here’s where it gets confusing: the higher the acidity, the lower the pH. So low pH means high acidity, and high pH means low acidity.
The big concern surrounding ocean pH boils down to human impact on ocean ecosystems. The carbon dioxide we produce through fossil fuel burning decreases the pH of our oceans. When carbon dioxide enters the ocean, be it natural or human-generated, it reacts with water to form carbonic acid. That breaks apart to form bicarbonate ions and hydrogen (H+) ions. When the concentration of H+ ions increases the pH of the ocean decreases.
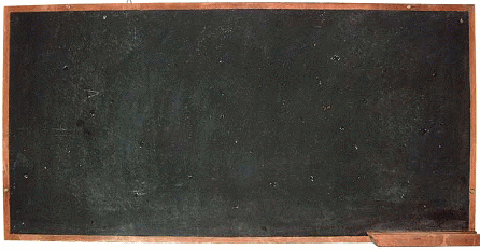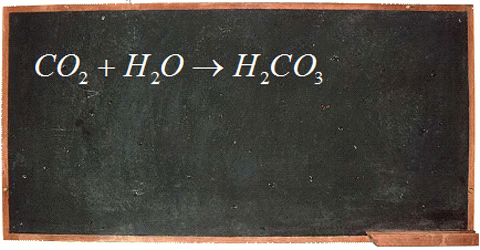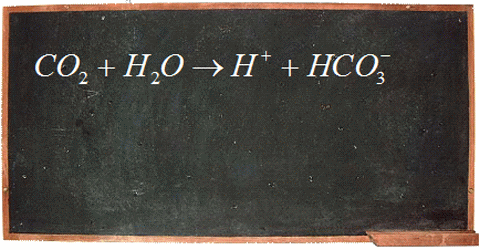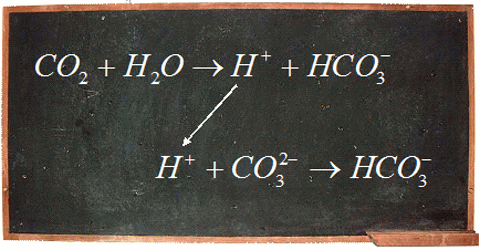
This is important because there is a narrow range of pH that is suitable for ocean ecosystems to thrive, and a changing ocean pH can have large effects on the organisms. For example, more acidified oceans have a lower abundance of carbonate ions, which are important building blocks in the shells and skeletons of marine organisms like oysters, clams, pteropods, corals, and phytoplankton. Without their shells these organisms are very vulnerable. Since shellfish and phytoplankton make up a large component of the marine food web, it doesn’t only affect them; it can affect the animals that feed on them and continue far up the food chain.
That is why my lab at University of South Florida College of Marine Science has joined the NOAA West Coast Ocean Acidification Cruise to measure pH off the west coast of the U.S. It is important to understand how pH is changing in these very productive areas so we can figure out how the ecosystem will respond, for better or for worse.
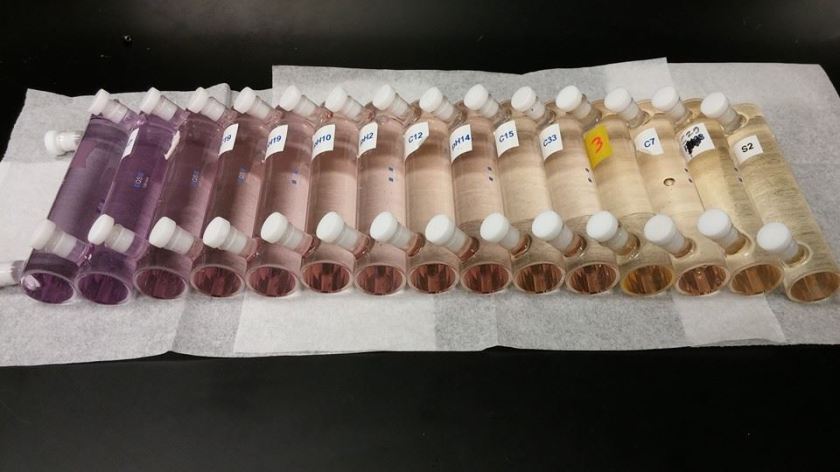
Our lab measures pH using an indicator dye. The indicator dye has a purple “basic” form and a yellow “acidic” form. Based on the pH of the seawater being measured, there will be different amounts of the basic and acidic forms and therefore the color of the seawater sample will turn yellow, purple, or some color in between. We use a very sensitive instrument called a spectrophotometer to measure the ratio of the acidic and basic forms of the dye. The spectrophotometer shines a light beam through the sample and measures the amount of light absorbed by each form of the indicator. They absorb light at different wavelengths so you can tell the difference between the two. We measure the ratio of the light absorbed by the acidic form and basic form of the dye and with a little mathematical magic we can calculate the pH of the seawater sample.

This method sounds complicated but the actual measurement process is very simple for the scientists onboard the ship. It just requires filling a glass cell with seawater, making sure the cell is wiped clean, adding a little indicator dye, and pressing some buttons on the spectrophotometer – all easy tasks. The software and the spectrophotometer do all the work. That is one of the benefits of this method; the other is state-of-the-art precision and accuracy. With a little practice you can measure about 18 samples per hour. That’s a good thing because by the end of this cruise we will have measured somewhere around 2000 samples at different depths and locations along our cruise! Our ship, the NOAA Ship Ronald H. Brown, will be sampling off the coast of Mexico, California, Oregon, Washington and even Canada. So after all the samples are analyzed at each of our 144 stations, we will have a better understanding of the acidity in marine habitats from the surface down to depths as deep as 2500 meters (about one and a half miles).
Author: Erin Cuyler


That’s a great report Erin ! Very interesting too. Safe travels!
Uncle Tom, Liz and Kate
LikeLike
Great article, Erin!
LikeLike
Really loved your article, Erin !
It made it easy to understand what I know is a very complex thing.
Keep us updated !
LikeLike
Great article! Really interesting and informative. You wrote it in a way that was easy to understand and the details really help with not only what you are doing, but why it’s important. Nice job!
LikeLike